Describe the Formation of Positive and Negative Ions
A positive ion and a negative ion are a part of an atom or a molecule. They are formed when atoms gain or lose electrons in order to fulfill the octet rule.
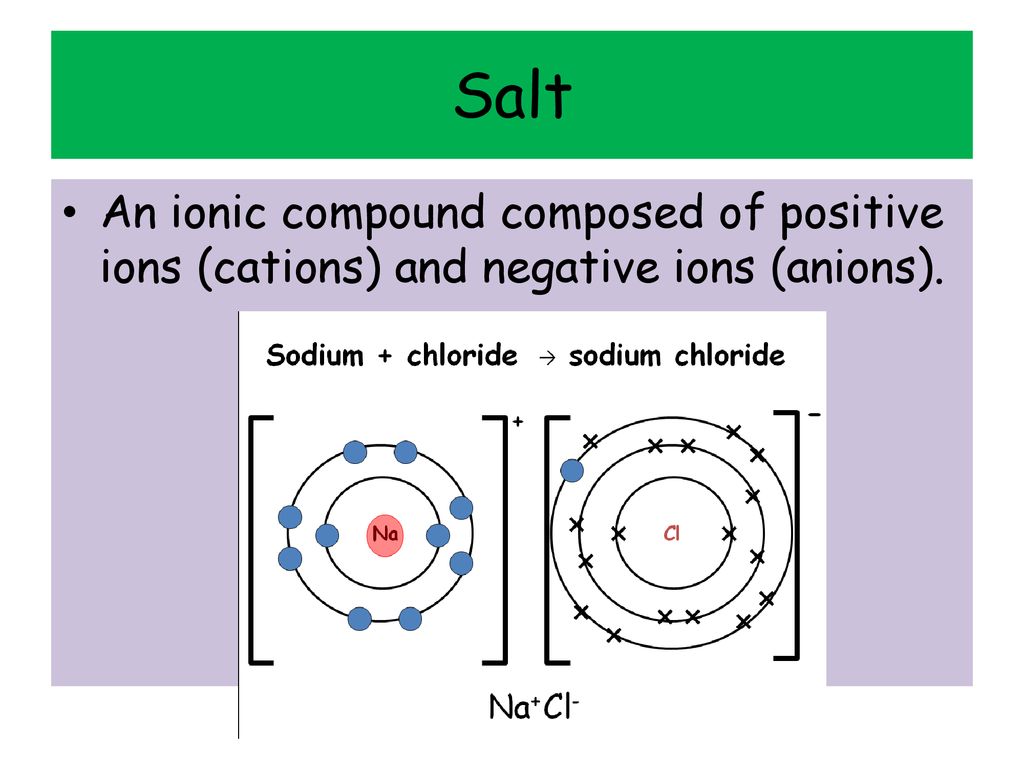
Positive Ions Charge Negative Ions Ammonium Nh4 1 Chloride Cl 1 Ppt Download
In chemistry atom is considered to be the smallest particle of a matter.

. A negative ion anion is formed when an atom or group of atoms gains electrons until there are more electrons than protons. Ions bound together by electrostatic attraction form ionic crystals. Describe the formation of positive and negative ions.
The key difference between the two ions is their net electrical charge of the ion wherein a positive ion has a net negative charge and a negative ion has a net positive charge. A negative ion also known as anion is formed when an atom or group of atoms gains electron s until there are more electrons. Describe the formation of ions by electron loss or gain Ions are electrically charged particles.
Elements gain or lose electrons to form ions and to gain full outer shells. For example lets look at Lithium and Fluorine. Compare the stability of a lithium atom with that of its ion Li.
A chemical bond is the force that holds two atoms together. Electrons are a form of positive ions and protons are a form of negative ion. Cations positively-charged ions and anions negatively-charged ions are formed when a metal loses electrons and a nonmetal gains those electrons.
An ion is defined as an atom or group of atoms where the number of electrons is not equal to the number of protons. When an atom gains electrons a negative ion is formed. Describe two different causes of the force of attraction in a chemical bond.
It positive ion because it contains more proton than electron. Form negative ions through electron gain except for hydrogen. A positive ion also known as cation is formed by the removal of electron s from an atom or group of atoms until there are fewer electrons than protons.
A positive ion cation is formed by removing electrons from an atom or group of atoms until there are fewer electrons than protons. When an atom loses electrons this results in a positive charge. Multiplying a positive number by a negative number or a negative number by a positive number results in a negative resultFor addition if you add two positive numbers the result is positive.
The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound such as sodium chloride. The two causes are the attraction between the positive nucleus of one atom and the negative. They are formed from atoms by electron loss or gain.
Model Draw models to represent the formation of the positive calcium ion and the negative bromide ion. When positive and negative ions meet it results in electro-chemical change. This ions are regularly arranged in what is.
A simple cubic crystal lattice has ions equally spaced in 3D at 90 angles. To obtain a full outer shell. Ions are formed when atoms gain or lose valence electrons to achieve a stable octet electron configuration.
Find step-by-step Chemistry solutions and your answer to the following textbook question. Electrons have a negative charge whereas protons have a positive charge. When these two types of ions meet it results in the destruction of atoms in the process of electrons leaving a positively charged atom and becoming a negatively charged atom.
The first things we need to understand are the molecules themselves. Positive ions because the way in which our environment and our bodies react to them are vastly different. In ionic compounds compounds formed through ionic bonding the strong attractive forces between the positive and negative ions result in the formation of a giant ionic structure.
Their arrangement varies depending on the ions sizes or the radius ratio the ratio of the radii of the positive to the negative ion. When thinking about ions its good to know the difference of negative ions vs. Form positive ions through electron loss.
This type of ion is called an anion. When an atom gains electrons this results in a negative charge. Fluorine would gain this electron to have 10 electrons giving it 2 full outer shells.
Positive and Negative Ions. Describe the lattice structure of ionic compounds as a regular arrangement of alternating positive and negative ions exemplified by the sodium chloride structure. Ions form when atoms lose or gain electrons.
WOW - Ionic Compounds and Metals Ionic Compounds and Metals Ion Formation. Chemical bonds form by the attraction between the positive nucleus. The charge on an ion is due to the unequal number of protons and electrons present in its atom.
Describe how ions form ionic crystals. 4 rows Forming negative and positive ions Forming negative ions anions Atoms gain electrons in. When an atom loses electrons a positive ion is formed.
They form through Ionic bonding. The Li ion is more stable because it has a complete octet. Solution for Describe the formation of positive and negative ions.
Draw a shell model for boron B. Identify the core and valence electrons. A metal reacts with a nonmetal to form an ionic bond.
Lithium has 3 electrons so it needs to lose one to gain a full outer shell. Metal atoms lose electrons to form positively charged ions non-metal atoms gain. Calcium atom loses two electrons forming mathrmCa2.
All atoms around us typically have the same amount of protons and neutrons. Positive and negative ions are natural phenomena which are occurring in every instance around us. Describe the formation of positive and negative ions.

Ions And Ionic Bonding Igcse Chemistry Cambridge Cie Teaching Resources Ionic Bonding Chemistry Gcse Chemistry

Positive Negative Ions Formed 1 Ionic Bonding Ionic Ap Chemistry

Difference Between Positive And Negative Ion Compare The Difference Between Similar Terms
Comments
Post a Comment