The C Cl Bond Is Best Described As___________
At carbon and This problem has been solved. The Cl-Cl bond of chlorine is best described as Question The Cl-Cl bond of chlorine is best described as Transcribed Image Text.

Solved Your Answer Is Incorrect Try Again The Cl Ci Bond Of Chegg Com
8 at carbon and S-at chlorine B ionic C nonpolar.

. The C-C bond of chlorine is best described as Nonpolar. Which of the following statements best describes the C-Cl bond in the following compound. The ClCl bond is best described as a Nonpolar covalent b Polar covalent c Ionic from CHE 2401 at Gordon College.
Induction and Polar Covalent bond. The C-Cl bond is best described as A. Which of the following statements best describes the CCl bond in 1-chloropropane.
HH cic C- H HHHH A polar. An ionic bond is best described as a attraction. At carbon and ionic polar.
E a bond between a metal and a polyatomic ion. There is extended conjugation present in option d which will reduce the length of C-Cl bond to the greatest extent which can be represented as follows. The C-Cl bond is best described as O Nonpolar covalent Polar covalent O Ionic O Coordinate covalent.
The distribution of electrons between atoms. The CCl bond is best described as___________. The Cl Cl bond is best described as A nonpolar covalent B polar covalent C ionic from CHM 241 at Northern Virginia Community College.
D Cl-CHCH-NO 2 Solution. It is best described as _____. A Nonpolar covalent B Polar covalent C Ionic D Coordinate covalent E None of theseAns.
Nonpolar covalent Polar covalent Ionic Coordinate covalent None of these. The bond between two polyatomic ions. How many distinct p orbitals exist in the 2nd electron shell.
The CCl bond is best described as___________. The C-Cl bond is best described as Nonpolar covalent Polar covalent Ionic O Coordinate covalent O None of these Identify the relationship between the following two. It is the compound of non polar covalent bond.
It is a diatomic molecule of chlorine. What is the expected bond angle for. The electron configuration of a carbon atom has how.
The electronegativity values for. Draw the Lewis structure of CHCl₃ and then determine the ideal bonding. Trigonal planar 120 degsp2.
50 the c cl bond is best described as a nonpolar. The C-Cl bond is best described as Nonpolar covalent Polar covalent Ionic O Coordinate covalent O None of these Identify the relationship between the following two. The Cl-Cl bond of Chlorine is best described how.
Chloroform CHCl₃ is an important laboratory solvent with a relatively high vapor pressure at room temperature. For the methyl cation CH3 the geometry H-C-H bond angle and hybridization of the carbon atom is best described as. Answered The Cl-Cl bond is best described as___________.
A chemical bond formed when two atoms share one pair of electrons is a _____ bond. A Nonpolar covalentB Polar covalent C Ionic D Coordinate covalent E None.
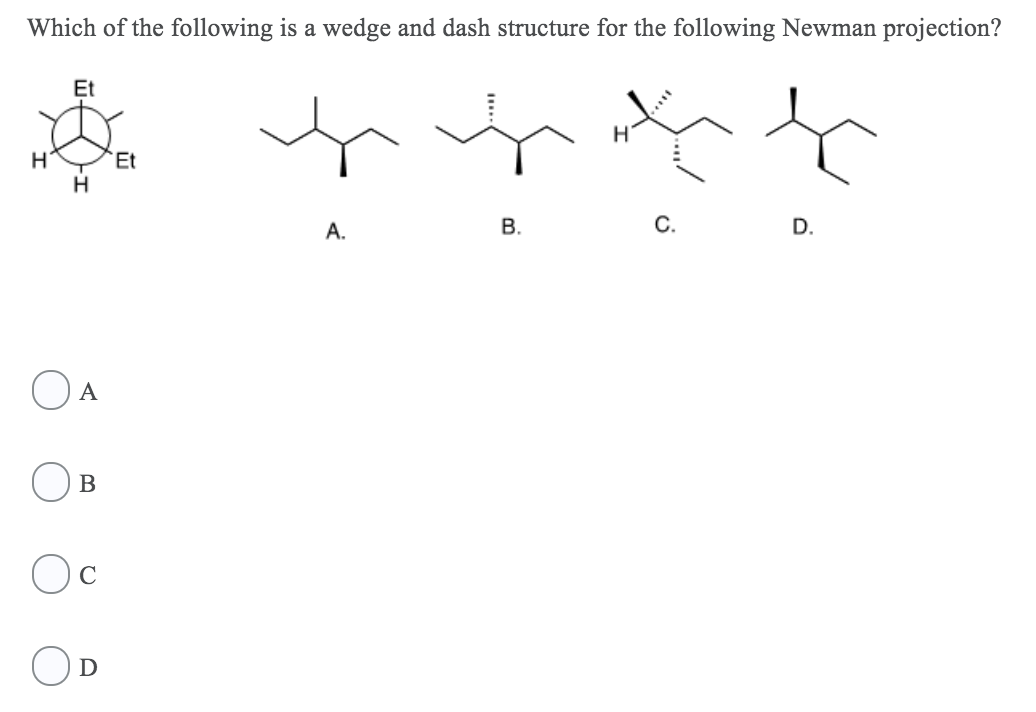
Solved The C Cl Bond Is Best Described As Nonpolar Covalent Chegg Com
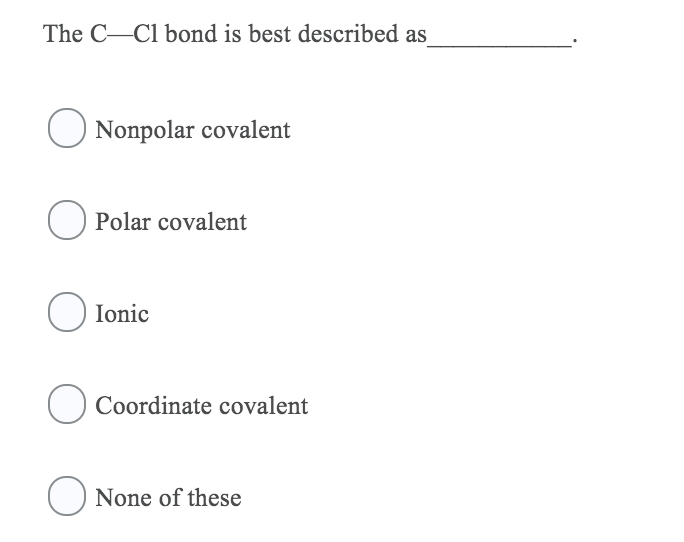
Solved The C Cl Bond Is Best Described As Nonpolar Covalent Chegg Com
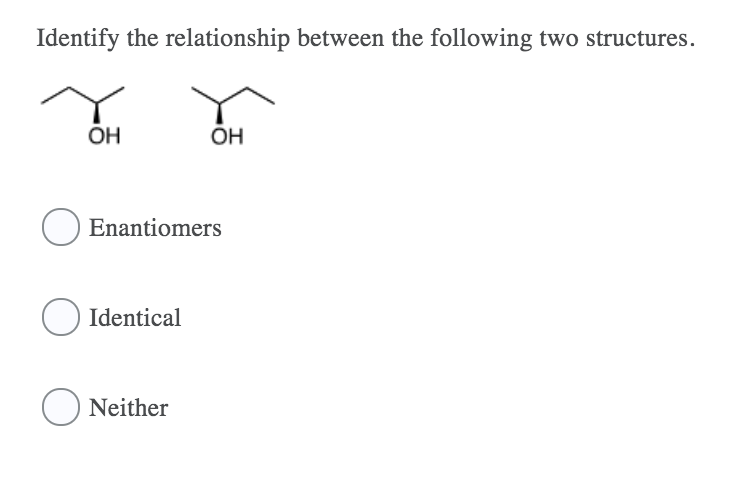
Solved The C Cl Bond Is Best Described As Nonpolar Covalent Chegg Com
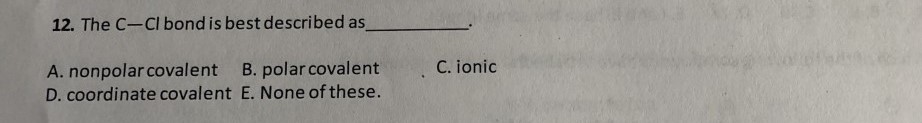
Usutmek Indirim Yutak C Cl Polar Or Nonpolar Holzkugelbahn Net
Comments
Post a Comment